Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kokubu, Yoko; Matsushi, Yuki*; Ishizaka, Chika*; Hirao, Noriaki*; Yonaga, Yusuke; Yoshikawa, Kiyotaka*
JAEA-Testing 2019-002, 101 Pages, 2019/11
This report provides a description of sample preparation method for measurement of in-situ belliyum-10 ( Be) and allumium-26 (
Be) and allumium-26 (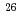 Al) in terrestrial quartz using accelerator mass spectrometry at Tono Geoscience Center, Japan Atomic Energy Agency. The report is based on the laboratory manual "Chemistry for in-situ
Al) in terrestrial quartz using accelerator mass spectrometry at Tono Geoscience Center, Japan Atomic Energy Agency. The report is based on the laboratory manual "Chemistry for in-situ  Be and
Be and 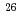 Al measurement for terrestrial quartz by AMS at MALT ver.1.3 and ver.2.2" prepared by ph.D Matsushi at Kyoto University.
Al measurement for terrestrial quartz by AMS at MALT ver.1.3 and ver.2.2" prepared by ph.D Matsushi at Kyoto University.
Aramaki, Takafumi; Mizushima, Toshihiko; Mizutani, Yoshihiko*; Yamamoto, Tadatoshi; Togawa, Orihiko; Kabuto, Shoji*; Kuji, Tomoyuki*; Gottdang, A.*; Klein, M.*; Mous, D. J. W.*
Nuclear Instruments and Methods in Physics Research B, 172(1-4), p.18 - 23, 2000/10
Times Cited Count:26 Percentile:82.56(Instruments & Instrumentation)no abstracts in English
; *; ; ; Aoto, Kazumi;
JNC TN9400 2000-059, 43 Pages, 2000/05
The purpose of this study is to understand the material properties of manufacturable high-purity iron and high-purity iron-based alloy in present technology and to get an applicable prospect for the structural and functional material of the frontier fast reactor. Then the about 10kg high-purity iron and iron-based alloy were melted using a cold-crucible induction melting furnace under the ultra-high vacuum. Subsequent to that, the compatibility between the melted material and the high-temperature sodium environment which is a special feature of the fast reactor and tensile property at room and elevated temperatures were investigated using the melted materials. Also, the creep test using the high-purity 50%Cr-Fe alloy at 550 C in air in order to understand the high temperature creep property. ln addition, the material properties such as thermal expansion coefficient, specific heat and electrical resistance were measured and to evaluate the outlook for the structural material for the fast reactor. The following results were obtained based on the property test and evaluation. (1)lt was possible to melt the about 10kg high-purity ingot and high-purity 50%Cr-Fe alloy ingot using a cold-crucible induction melting furnace under the ultra-high vacuum. (2)The tensile tests of the high-purity 50%Cr-Fe alloy were performed at room and elevated temperatures in order to understand the deformation behavior. From the experimental results, it was clear that the high-purity 50%Cr-Fe alloy possesses high strength and good ductility at elevated temperatures. (3)The physical properties (the thermal expansion coefficient and specific heat etc.) were measured using the high-purity 50%Cr-Fe alloy. lt was clear that the thermal expansion coefficient of high-purity 50%Cr-Fe alloy was smaller than that of SUS304. (4)From the corrosion test in liquid sodium, the ordinary-purity iron showed the weight loss after corrosion test. However the high-purity iron showed ...
C in air in order to understand the high temperature creep property. ln addition, the material properties such as thermal expansion coefficient, specific heat and electrical resistance were measured and to evaluate the outlook for the structural material for the fast reactor. The following results were obtained based on the property test and evaluation. (1)lt was possible to melt the about 10kg high-purity ingot and high-purity 50%Cr-Fe alloy ingot using a cold-crucible induction melting furnace under the ultra-high vacuum. (2)The tensile tests of the high-purity 50%Cr-Fe alloy were performed at room and elevated temperatures in order to understand the deformation behavior. From the experimental results, it was clear that the high-purity 50%Cr-Fe alloy possesses high strength and good ductility at elevated temperatures. (3)The physical properties (the thermal expansion coefficient and specific heat etc.) were measured using the high-purity 50%Cr-Fe alloy. lt was clear that the thermal expansion coefficient of high-purity 50%Cr-Fe alloy was smaller than that of SUS304. (4)From the corrosion test in liquid sodium, the ordinary-purity iron showed the weight loss after corrosion test. However the high-purity iron showed ...
; ; ; Ikeda, Hisashi ; Jitsukata, Shu*; *
JNC TN8410 2000-022, 55 Pages, 2000/05
Measurement of U and Pu concentrations by wavelength dispersion type X-ray fluorescence spectrometry was studied. Sample holder was installed inside of glove box and other instruments, X-ray tube, monochromator and detectors were set out side of the glove box. X-rays was irradiated to sample though Be window. Fluorescent X-rays form sample were also passing though the same Be window and detected outside. Analytical conditions were optimized as follows. Sample thickness is 8 mm, which is 3ml of sample volume by the sample holder. Voltage and eurrent for X-ray tube is 50kV and 40 mA, respectively. Measurement was done twice, 60 seconds each, and averaged X-ray intensity was used to calculate elemental concentrations. Matrix correction was necessary to measure U and Pu concentration within 10% accuracy. Detection limits were calculated to 0.4 mg/L for U and 0.7mg/L for Pu. Calibration curve was liner up to 9 g/L fbr U and Pu. Two calculation methods, calibration curve method and standard addition method, were studied to measure Pu concentration in organic solution. Detection limit was 5.3 mg/L and 0.2 mg/L, respectively.
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...
Usuda, Shigekazu
Proceedings of International Workshop on the Status of Measurement Techniques for the Identification of Nuclear Signatures, p.237 - 244, 1997/00
no abstracts in English
*; *;
Radioisotopes, 30(9), p.475 - 479, 1981/00
no abstracts in English
*;
Int.J.Appl.Radiat.Isot., 31, p.619 - 622, 1980/00
Times Cited Count:5 Percentile:53.45(Nuclear Science & Technology)no abstracts in English
Bunseki Kagaku, 14(3), p.271 - 275, 1965/00
no abstracts in English
 O-B
O-B O
O system
systemNakajima, Kunihisa; Takai, Toshihide; Furukawa, Tomohiro; Osaka, Masahiko
no journal, ,
There are limited thermodynamic data available for compounds in the Cs-B-O system. We have a plan to evaluate the thermodynamic properties of the cesium borates from their equilibrium vapor pressures determined by a Knudsen effusion mass spectrometry. Cesium borates such as triborate, CsB O
O , were prepared and phase relations in the Cs-B-O system were investigated using a powder X-ray diffractometer. As one of the results, it was found that CsB
, were prepared and phase relations in the Cs-B-O system were investigated using a powder X-ray diffractometer. As one of the results, it was found that CsB O
O has an orthorhombic crystal structure.
has an orthorhombic crystal structure.